
Aug 1
How an Israeli executive built a network inside Seattle's government
Glen Stellmacher, Special to the SGN READ TIME: 2 MIN.
Editor's Note: The contents herein are provided for informational purposes only and shall not be construed to imply any illegality or misconduct by any organization or individual, including employees or officials acting on behalf of the City of Seattle. All graphics are for illustrative purposes only. Any logos or individuals depicted are not intended to imply endorsement of the content presented herein.
The Advanced Security Training Institute (ASTI), a 501(c)(3) non-profit that trains American police inside Israel, has previously liked numerous X (Twitter) posts that have expressed anti-LGBT and anti-immigrant views, according to a review of its X account by the SGN.
The president and founder of ASTI, Yisroel Stefansky, has also exchanged numerous private LinkedIn messages with the director of Seattle’s Office of Emergency Management (OEM), Curry Mayer.
Some of Mayer’s LinkedIn messages with Stefansky were provided to the SGN through a public records request. They show Stefansky calling Mayer “my dear friend” and saying, “Let me know what I should bring you from Jerusalem.” Seattle ethics laws prohibit city employees from receiving gifts that “would appear to a reasonable person to have been given, solicited, or received with the intention to give or obtain special consideration or influence.”
Mayer did not appear to respond to Stefansky’s offer.
The SGN is aware of at least 13 messages between Mayer and Stefansky that have not been disclosed, by comparing emails Mayer received that indicate she received a LinkedIn message from Stefansky and the absence of those messages from the city’s public records disclosure. After the SGN appealed the closure of a city public records request asking for Mayer’s LinkedIn messages, the City wrote that “communications on personal accounts regarding purely personal matters are not within the scope of employment. They are not public records, and therefore not responsive to this request.”
The SGN has since asked Mayer directly if she would disclose all of her personal LinkedIn messages with Stefansky but has not received a response. Stefansky and Mayer have not responded to multiple requests for comment.

Stefansky’s ASTI corporation offers American law enforcement, emergency management, and fire department officials training in Israel, taught by Israeli instructors. However, Stefansky also appears to travel to the United States and has even offered events in Seattle.
In 2022, Mayer helped to organize an ASTI event at Seattle City Hall with a former official of Israel’s national security agency, known colloquially as Shin Bet or Shabak and officially as the Israel Security Agency (ISA).
Records obtained by the SGN show that in January 2024, Stefansky also emailed Mayer a prospectus of ASTI’s latest law enforcement training courses offered in Israel, including lessons on Israeli history, “contested territories,” and “combatting domestic violent extremism.”
Mayer herself is not the first Seattle city employee to communicate with Stefansky, who has run law enforcement training junkets to Israel for multiple Seattle Police Department (SPD) officers. Stefansky also appeared to serve as a personal tour guide for former Seattle Mayor Ed Murray’s junket to Israel in 2015. At the time, activist critics labeled Murray’s trip "pinkwashing," meaning to promote a pro-gay public image of Israel to distract from Israel's systemic human rights abuses against Palestinians. Journalist Guy Oron has also reported on a junket Stefansky led to Israel with former US Rep. Dave Reichert in 2013.

ASTI’s social media footprint
ASTI’s X account has since engaged with highly controversial, conspiratorial, and inflammatory content, indicative of a pattern of favor toward the right-wing political movement and its associated ideological grievances against the LGBTQ and immigrant communities.
A prior version of the social media app X allowed any user to view any other user’s liked content. After Elon Musk purchased the company, the platform removed this feature in June 2024 and has since hidden public access to other users’ liked posts.
However, prior to this change, the SGN reviewed numerous X posts liked by ASTI, including a potpourri of inflammatory theories, including claims that UNRWA is a money-laundering front for Hamas, and that Minneapolis Police Officer Derek Chauvin’s murder trial of George Floyd was an “absolute sham.” ASTI has liked X posts calling to pay all January 6 “hostages” reparations and to “start locking up the Democrats.”
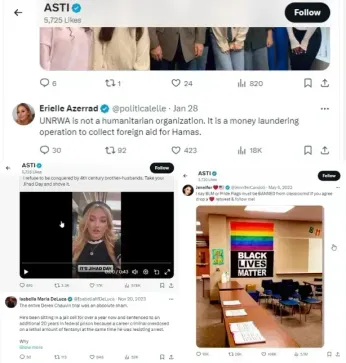
One post liked by ASTI included text over a Pride/rainbow flag that said, “If they have the right to fly theirs,” then, over a Confederate battle flag, “we deserve the right to fly ours.”
Other posts liked by ASTI include one claiming that Pride flags have ruined Kansas by wokeness; another calling the Trans Day of Visibility a “fucking joke”; one saying “This entire, like, transgender stuff at this point honestly feels like a huge joke”; a video of a woman ripping down a rainbow Pride flag and throwing it in the trash; a post by Sebastian Gorka calling a video of an activist advocating for LGBTQ kids at school a “mental disease”; and a since-deleted post saying “BLM or Pride flags must be BANNED from classrooms.”
Frequently, ASTI has liked content that refers to people as “ILLEGALS,” including a video of people standing in line to be legally processed with US Customs and Border Protection, labeled “illegal… military aged men.” A number of posts liked by ASTI called for “mass deportations.” Another ASTI-liked post included an image that reads “America’s top export should be illegal immigrants.”

Concerns
Dean Spade, a professor at the Seattle University School of Law and the author of Mutual Aid: Building Solidarity During this Crisis (and the Next) and Love in a F*cked Up World, told the SGN that “it's disturbing to see the content that ASTI liked, yet not surprising to see alignment of the security industry, Zionism, attacks on Trans people and immigrants, promotion of police violence, and other themes represented in those posts. It is important that people in cities like Seattle, where the city government portrays itself as liberal, antiracist, and inclusive, understand the unsavory alliances that actually undergird the city's police force and city government relationships with surveillance and security industries.”
Prof. Spade told the SGN that he is “not surprised when public officials fail to be transparent, but I do think we should be concerned about Seattle public officials' connections to right-wing figures like Stefansky. These people explicitly seek to find foothold within city governments to expand the scope of their influence and sell ever more of their dangerous wares. The influence of security industry conservatives on Seattle city officials is certainly a threat to people of color, immigrants, Queer and Trans people, poor people, and people with disabilities in the city.”
Wendy Elisheva Somerson, also known as Wes, one of the founders of the Seattle chapter of Jewish Voice for Peace (JVP), told the SGN that Mayer’s private channel of communication with Stefansky is reprehensible.
"The advocates for Zionism and the Israeli state are not really interested in the well-being of Queer people," Somerson said. "To see these homophobic, transphobic, and racist posts that ASTI is liking, I will admit there is no surprise."
Spade told the SGN that “the people who will be labeled extremists, yet again, are those advocating against racism, and for justice in Palestine, justice for migrants, and justice for Trans people. The SPD already targets people in liberation movements, and further training of this kind will bring us even closer to the conditions visible in Israel, where law enforcement brutally enacts a colonial agenda of surveillance and violence. Our police don't need more of this kind of training. Instead, Seattle still needs to defund police and focus on real solutions to community crises around poverty, housing and health care access, childcare, and other essentials.”

Former Mayor Ed Murray’s Israel trip
Prior to his conversations with Seattle’s OEM Director, Stefansky was also involved in former Seattle Mayor Ed Murray’s trip to Israel in 2015.
The trip itself was reportedly funded and developed by the Israeli Ministry of Foreign Affairs. In the Seattle media, it was packaged as a “trade delegation” and a business exchange, punctuated by Murray’s keynote speech at the “40 Years of Pride” conference in Tel Aviv. A list of members of Murray’s delegation included Seattle city employees and private venture capitalists.
Withheld from the public sphere was that Murray’s administration intended to “engage in knowledge-sharing” with the Israelis, including meetings with the Israeli security sector.
Israel’s consulate general in San Francisco proposed that Seattle officials meet with a variety of private Israeli surveillance and military technology companies and government agencies, in addition to Israeli national fire, police and prison services.
“Since security/emergency management is one of the sectors we're focusing on as we work to form a delegation to accompany the mayor, we are reaching out to SFD, SPD, and OEM to see if there are any in the city that might want to take advantage of this opportunity to learn from Israel's expertise and share knowledge related to security issues,” wrote Seattle’s International Programs Coordinator Stacey Jehlik in 2015 before the trip.

Jehlik told the SGN that Stefansky “had no role in nor was he consulted on the 2015 trip agenda that I coordinated. It’s my understanding that he connected with members of the delegation that he knew outside of the trip scope and arranged for a meeting between Jerusalem fire officials and the Seattle Fire Department.”
Jehlik also told the SGN that Seattle’s Office of Intergovernmental Relations (OIR) is not currently planning any trips to Israel, nor is the OIR currently engaged in any active partnerships with the Israeli government.
A preliminary version of Murray’s itinerary also scheduled a meeting with Tal Becker titled “How Wide the Gap: Negotiations with the Palestinians.” At the time, Becker had been a legal advisor for the Israel Defense Forces (IDF) , the Israeli mission to the UN, and the Israeli Ministry of Foreign Affairs. As of early 2024, Becker is representing the state of Israel at the International Court of Justice (ICJ), where the country has been charged with genocide. Murray’s meeting with Becker was eventually canceled.
When Murray’s delegation landed in Israel in June of 2015, Stefansky was there to greet Seattle Fire Department Chiefs Rebecca Gonzalez and Charles Cordova. He led tours of attractions, alleys, and shops in Tel Aviv and in Jerusalem and took photos of Mayor Murray. No media outlet reported this connection at the time.

Seattle Police Department
A month after Murray returned from Israel, then Seattle Police Department (SPD) Deputy Chief Carmen Best (later serving as Chief of Police from 2018 to 2020) inked her signature on an agreement to attend an Anti-Defamation League (ADL) “National Counter-Terrorism Seminar” in Israel in September of 2015. Best’s attendance at this seminar was previously reported by End the Deadly Exchange Seattle, an offshoot group of Jewish Voice for Peace. (Seattle’s Be’er Sheva Sister City president then invited Chief Best to Israel again in 2018, with an explicit “opportunity to meet with your counterparts in the Israel Police.”)
However, Chief Best’s trip to Israel would not be the first time that SPD had sent a delegation to the country. Two SPD officers, Aaron Kamalu and Rodney Stokes, accompanied Mayor Murray on his 2015 trip, and prior to that, multiple SPD personnel had already attended training in Israel with Stefansky’s previous company, Proactive Global Security (PGS). Records previously reported by Guy Oron show that SPD officers Erik Allen and Timothy Renihan traveled with PGS to Israel in 2013 and Rik Hall with Shane Anderson traveled with PGS to Israel in 2014.
Anderson and Hall have since worked as part of SPD’s Intelligence Unit, and both were engaged in surveilling Black Lives Matter protests in 2020, according to SPD emails reviewed by the SGN. Real Change reported that Hall served as an SPD officer for the FBI’s Joint Terrorism Task Force in 2020.

Former Seattle Fire Department Deputy Chief Jay Hagen had also attended training with PGS. In one internal email, Stefansky was characterized as “a good friend” of Chief Hagen, who appeared to have sent his daughter to meet Stefansky in Israel in 2014. In 2015, Stefansky offered to bring Hagen Israeli olive oil and wine.
Hagen even forwarded internal “need to know basis” emails to Stefansky when the mayor of Tel Aviv visited Seattle in November of 2015, months after Murray returned from the country.
Stefansky would also later try and recruit Chief Best and King County Sheriff Mitzi Johanknecht to attend ASTI training programs in Israel in 2019. Neither ended up participating in the 2019 trip.
"When JVP put a lot of pressure and exposed these trips that police officers in the US were making to Israel, exchanging these 'worst tactics,' the ADL very quietly shut down their ‘deadly exchanges,’" Somerson told SGN. "The City of Seattle often hides behind its liberal reputation, but if you look behind the scenes with stuff like this, it's really disturbing."
The SPD did not respond to multiple requests for comment.

Prof. Spade told the SGN that “along with thousands of other people, I've been part of the End the Deadly Exchange campaign in Seattle and across the country, trying to stop local law enforcement from training in Israel, precisely because we can see how harmful it is for our already dangerous and racist local law enforcement to go train with armed forces in one of the most notoriously racist and genocidal countries in the world. It is a threat to the people of Seattle to have the SPD's power to surveil, hurt, and kill people enhanced through these training programs and collaborations.
“As conditions worsen in the US, with cuts to basic necessities, ecological crisis, and expanding state violence, our resistance movements are also ramping up. There is a long history of police infiltration of resistance movements and violence against people in our movements, and we are currently in a moment of increasing repression. It is essential to fight back against the expansion of surveillance and policing tactics, especially now with the growing crackdown on the Palestine solidarity and migrant justice movements.”
This is the first in a series entitled "Community Viewpoints," a space for vibrant conversations within Seattle's LGBTQIA+ community - exploring how we see ourselves, how the world sees us, and the impact we make together.
Support the Seattle Gay News: Celebrate 51 Years with Us!
As the third-oldest LGBTQIA+ newspaper in the United States, the Seattle Gay News (SGN) has been a vital independent source of news and entertainment for Seattle and the Pacific Northwest since 1974.
As we celebrate our 51st year, we need your support to continue our mission.
A monthly contribution will ensure that SGN remains a beacon of truth and a virtual gathering place for community dialogue.
Help us keep printing and providing a platform for LGBTQIA+ voices.
How you can donate!
Using this link: givebutter.com/6lZnDB
Text “SGN” to 53-555
Or Scan the QR code below!







